Oxford University Vaccine Could Be Available By 3rd November As Human Trials Well Underway
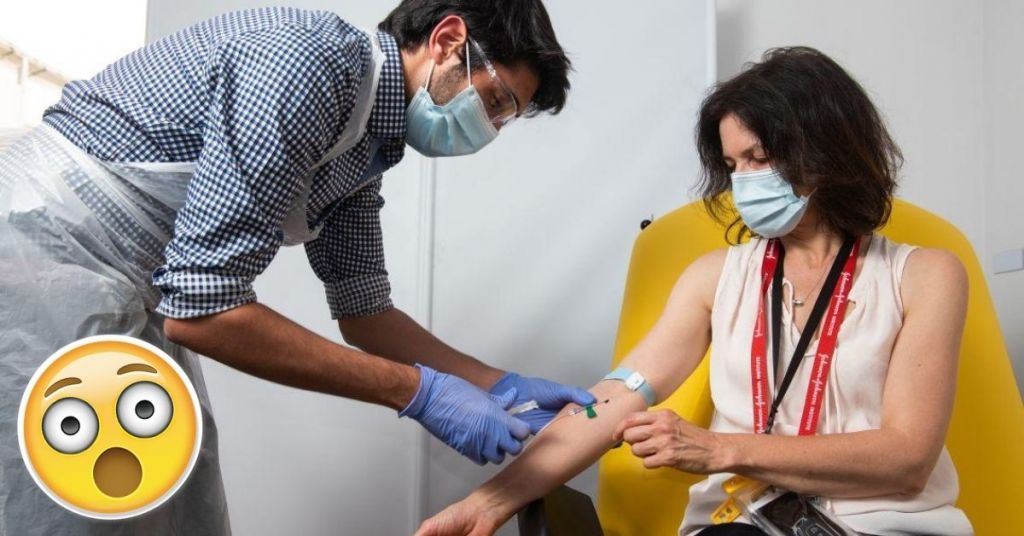
The COVID-19 vaccine being made by Oxford University and their collaborators AstraZeneca seems to be leading the vaccine efforts with their earliest date of distribution set for 3rd November.
After the successful trials of Phase 1 and 2, the company has begun Phase 3, human trials in India.
One of their manufacturing companies, the Serum Institute of India in Pune, has revealed that Phase 3 trials will only take 73 days so that would mean that means that by 3rd November vaccines would be available to the public.
“We are starting to manufacture this vaccine right now – and we have to have it ready to be used by the time we have the results,” said CEO of AstraZeneca to BBC reporters.
After this, the results will go through an assessment phase and if this comes up positive the license will be granted and the vaccines will begin being distributed.
The Institute has set an affordable ceiling price of US$3 per dose in an effort to make it more accessible to those in developing countries, they’ve also started a scheme on the lines of the same sentiments.
“SII has partnered with Gavi and the Bill & Melinda Gates Foundation to advance the manufacturing and delivery of up to 100 million doses of future COVID vaccines for India and low and middle-income countries in 2021,” said Adar Poonawalla, CEO of Serum Institute of India.
The human trials that are currently underway also seem to be successful with the first volunteer speaking out positively on the vaccine. This has encouraged people to take part in these trials also.
“We have been getting several calls from people wanting to enlist as volunteers,” said a top health official from the hospital caring for them.
